For H(2) gas, the compressibility factor,Z = PV //n RT is

For H(2) gas, the compressibility factor,Z = PV //n RT is
Which gas shows the maximum deviation from ideal gas, CO2 or NH3? Why? - Quora

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Compressibility factor of n-decane vapor (upper graph) and of ethylene
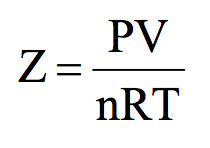
Real Gases - Chemistry, Class 11, States of Matter

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H
The given graph represent the variation of z compressibility factor z=pv/nRT versis p fpr three real gases A,B,C identify only incorrect statement
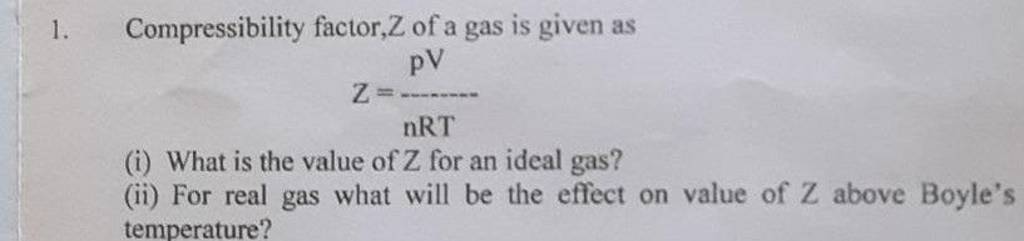
1. Compressibility factor, Z of a gas is given as Z=nRTpV (i) What is th..

Compressibility factor (gases) - Citizendium

Non-Ideal Gas Behavior Chemistry: Atoms First

Gas Compressibility Factor Calculator Excel SpreadsheetLow Cost

e Compressibility factor (Z) for hydrogen WRT pressure and temperature

Compressibility factor Z - Gaseous State

Solved The graph of compressibility factor (Z)v/sP for 1 mol
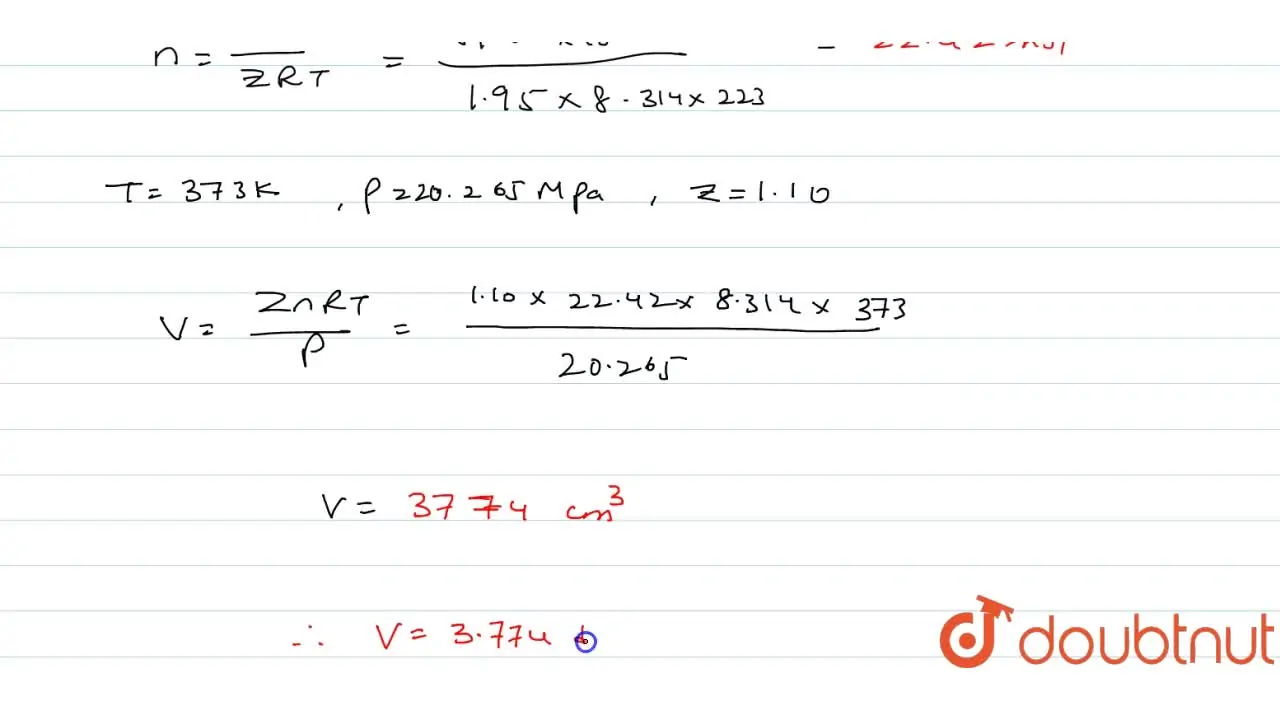
The compressibility factor (Z=PV//nRT) for N(2) at 223 K and 81.06 MPa