At 300 K, 36 g of glucose present per litre in its solution has an osm

pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"
Frontiers Dysnatremia in Gastrointestinal Disorders
What concentration of glucose is isotonic to human red blood cells? - Quora

At 300 K, 36 g of glucose present per litre in its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bar the same

HW PACKET 4 (pdf) - CliffsNotes

Find the molaity of water. Given: rho =1000kg//m^(3) [Report your

At 300K, 36g of glucose present in a litre of its solution has an osmotic pressure of 4.98bar.
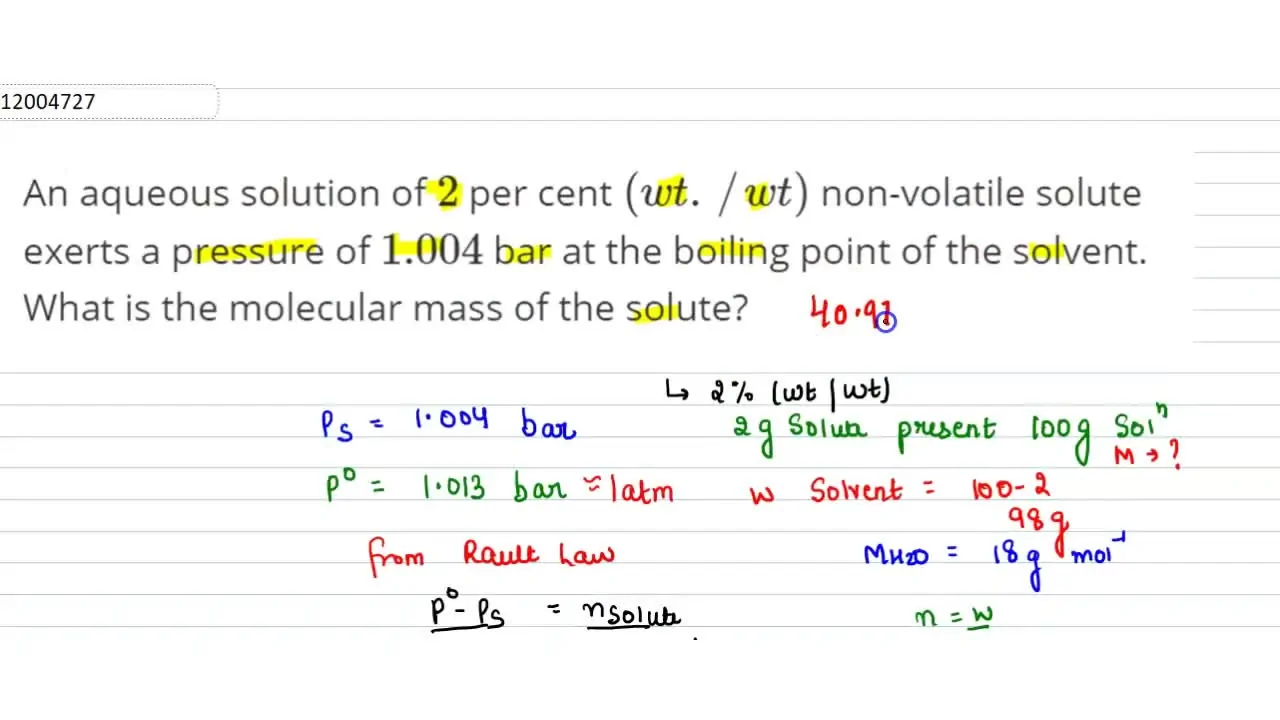
An aqueous solution of 2 per cent (wt.//wt) non-volatile solute exerts

⏩SOLVED:At 300 K, 36 g of glucose present per litre in its…

Kannada] At 300 K, 36 g of glucose present per litre in its solution

An antifreeze solution is prepared from 222.6 g of ethylene glycol [C(
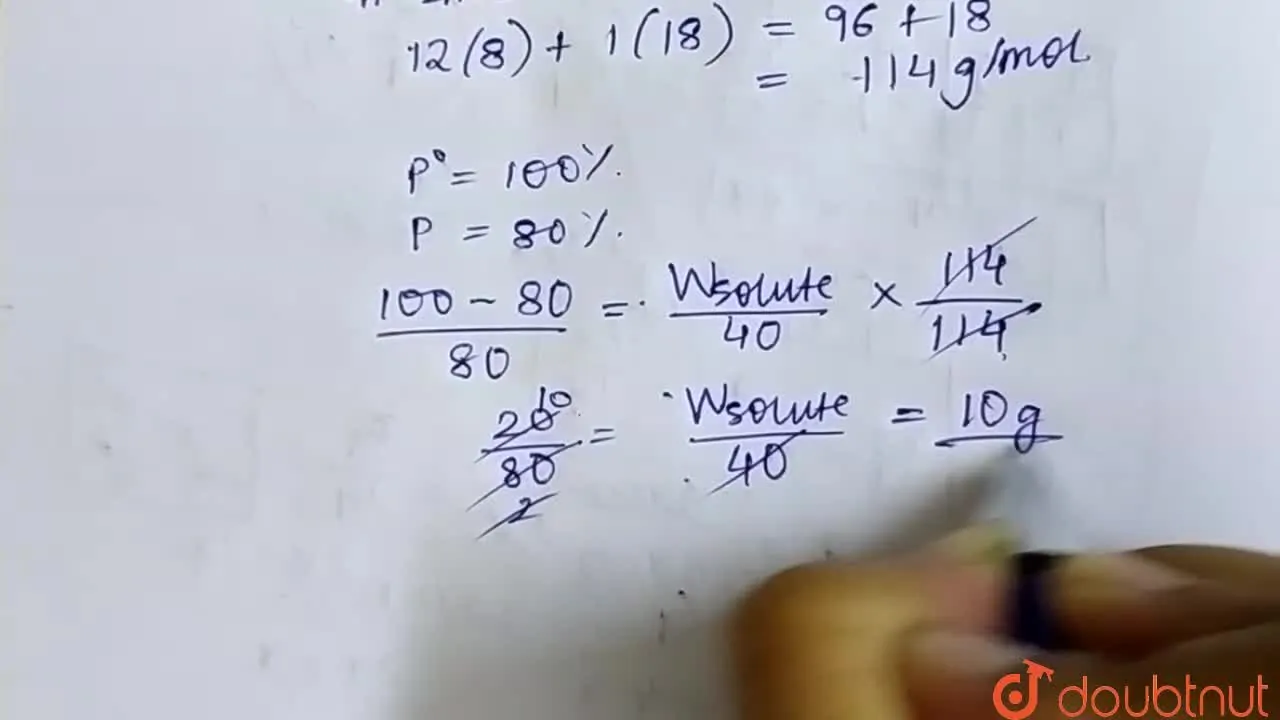
Calculate the mass of a non-volatile solute ( molecular mass 40) which

At 300K, 36g of glucose present in one litre of its solution has an os - askIITians

Chapter 13.5: Colligative Properties - Chemistry LibreTexts

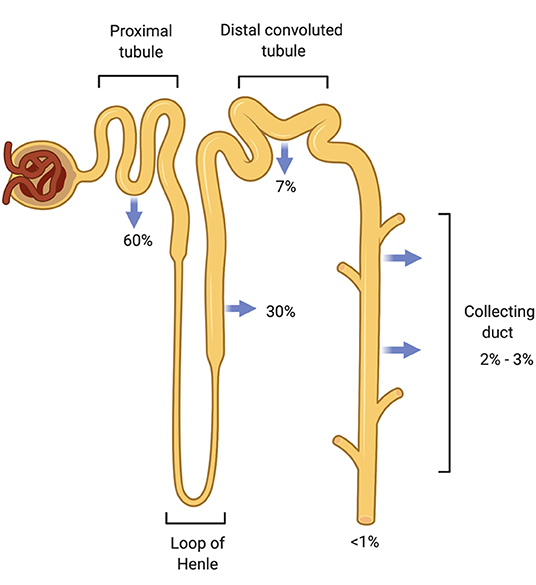
S2 Physiology Unit 2 - Body Fluid Physiology Flashcards







