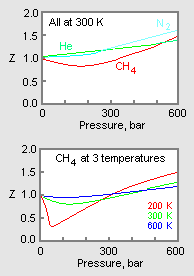
At high pressure, the compressibility factor 'Z' is equal toa)unityb) c) d)ZeroCorrect answer is option 'C'. Can you explain this answer? - EduRev NEET Question


A : At high pressure , the compressibility factor Z is (1 + (pb)/(RT))


For compressibility factor, Z, which of the following is /are correct?

Applying Concepts: Compressibility Factor, Chemistry
Why compressibility factor of areal gas is greater than unity at high pressure and temperature? - Quora

Solved 1) The compression factor, Z, can be written as: Z =
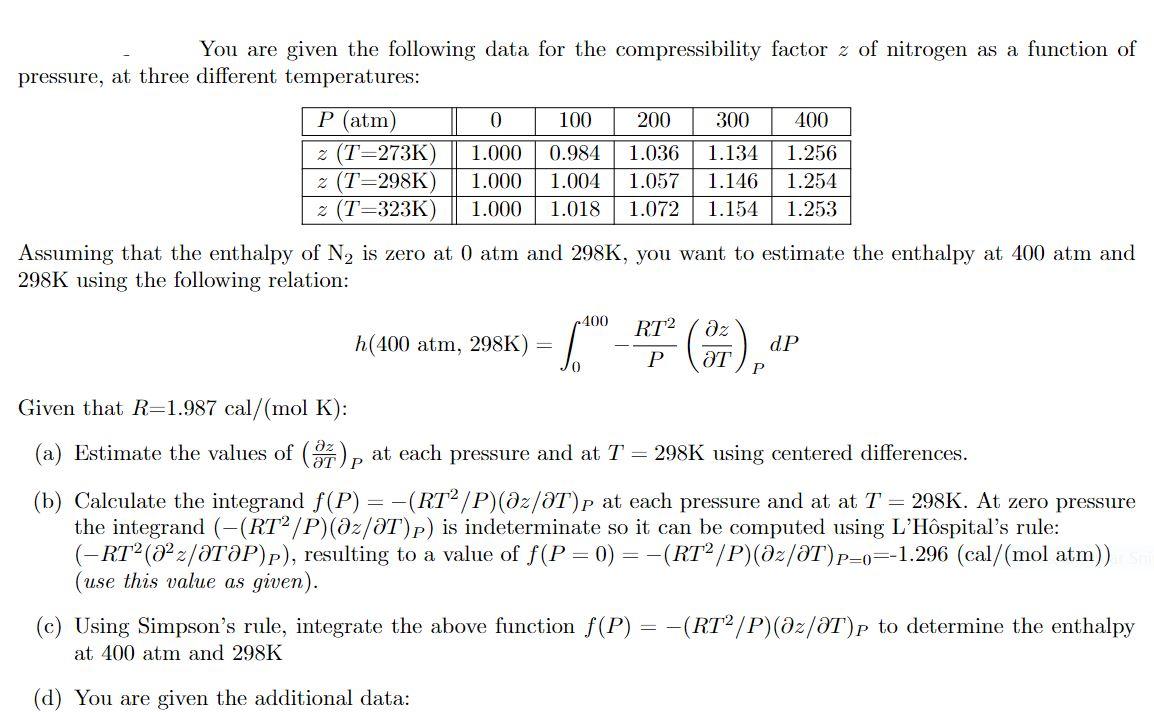
Solved You are given the following data for the

NEET 2019; Question Based on Compressibility Factor (Z); Previous Year Question Series

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

Compressibility Factor - an overview
:max_bytes(150000):strip_icc()/georgehqa_l-4cb965f07f084ce2924914b2187fb5ba.jpg)