In the following compressibility factor (Z) vs. pressure graph 300

Click here:point_up_2:to get an answer to your question :writing_hand:in the following compressibility factor z vs pressure graph at 300 k the compressibility of
Click here👆to get an answer to your question ✍️ In the following compressibility factor -Z- vs- pressure graph 300 K- the compressibility of CH-4- pressure - 200 bar deviates from ideal behaviour becauseThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is same as that in its ideal stateIntermolecular interactions between CH-4- molecules decreases
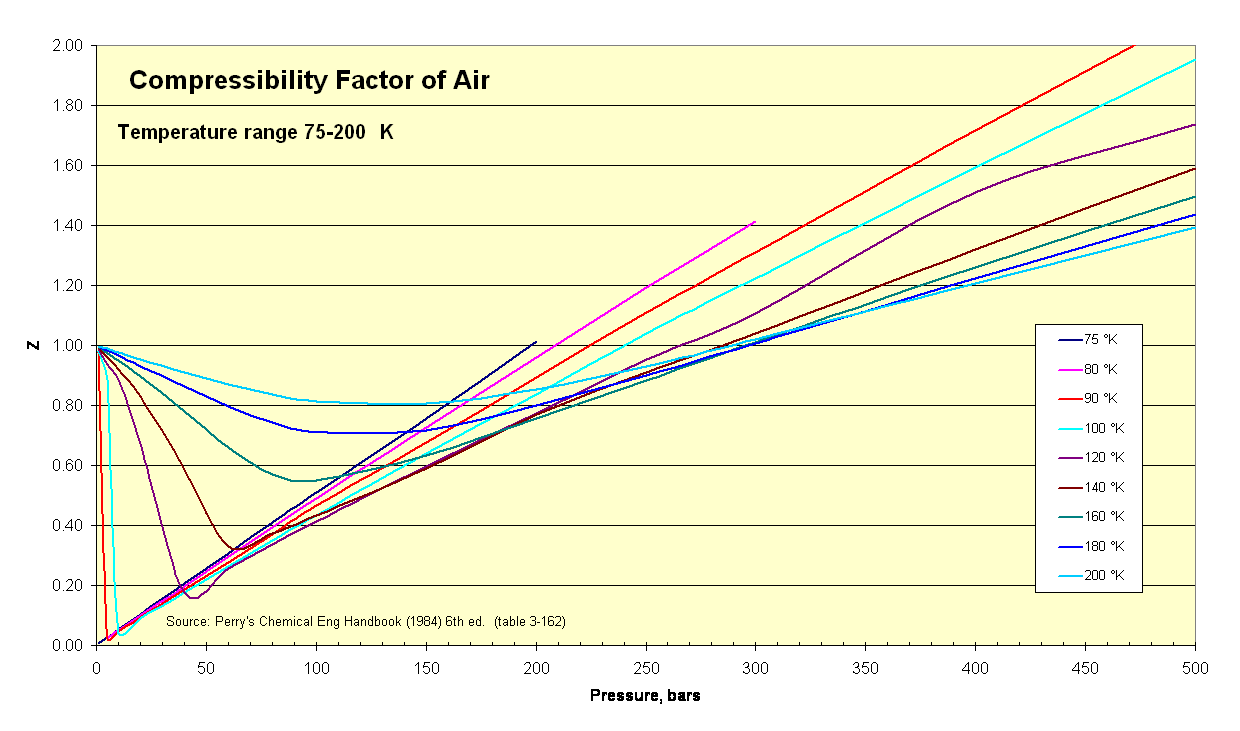
Air Compressibility Factor Table - EnggCyclopedia

gas laws - Graph of compressibility factor vs pressure when real gas is assigned Z=1 - Chemistry Stack Exchange

In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure

Energies, Free Full-Text
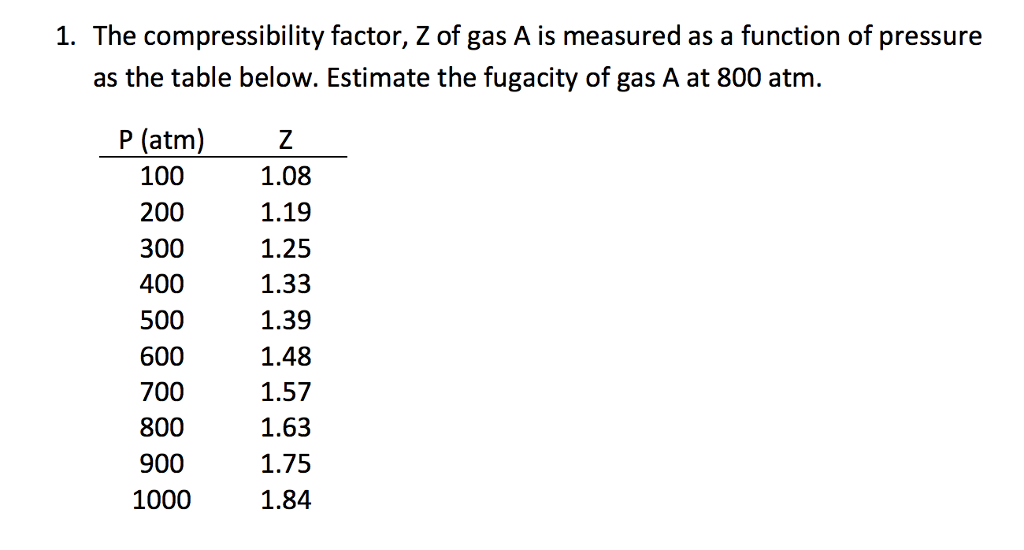
The compressibility factor, Z of gas A is measured as
Compressibility factor of water

Graph of Compressibility Factor (Z) versus Pressure (Atm)

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
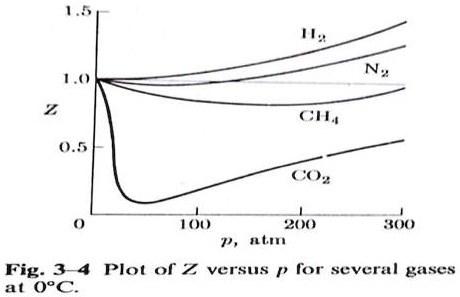
SOLVED: Subject: Compressibility Factor What is the analysis of the graph? 1.5 N 1.0 Z CHA 0.5 CO2 1 0 100 200 P, atm 300 Fig. 3-4: Plot of Z versus p for several gases at oc.
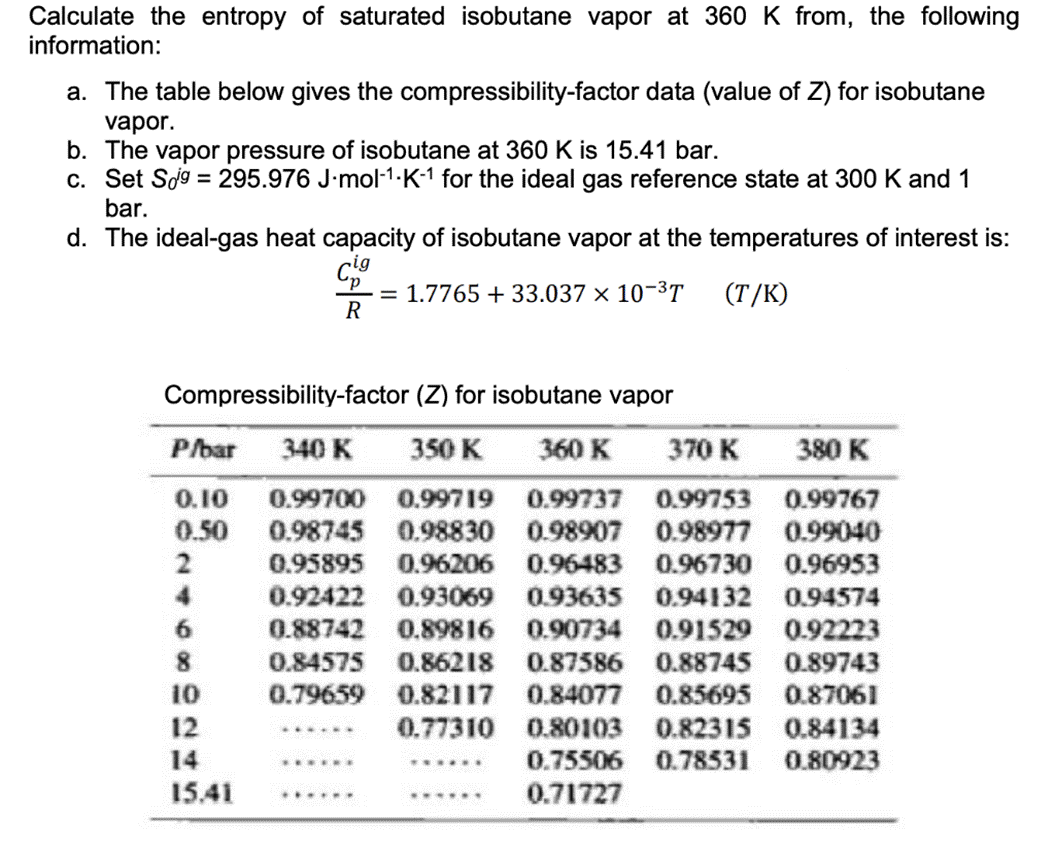
Solved) - Calculate the entropy of saturated isobutane vapor at 360 K from, (1 Answer)
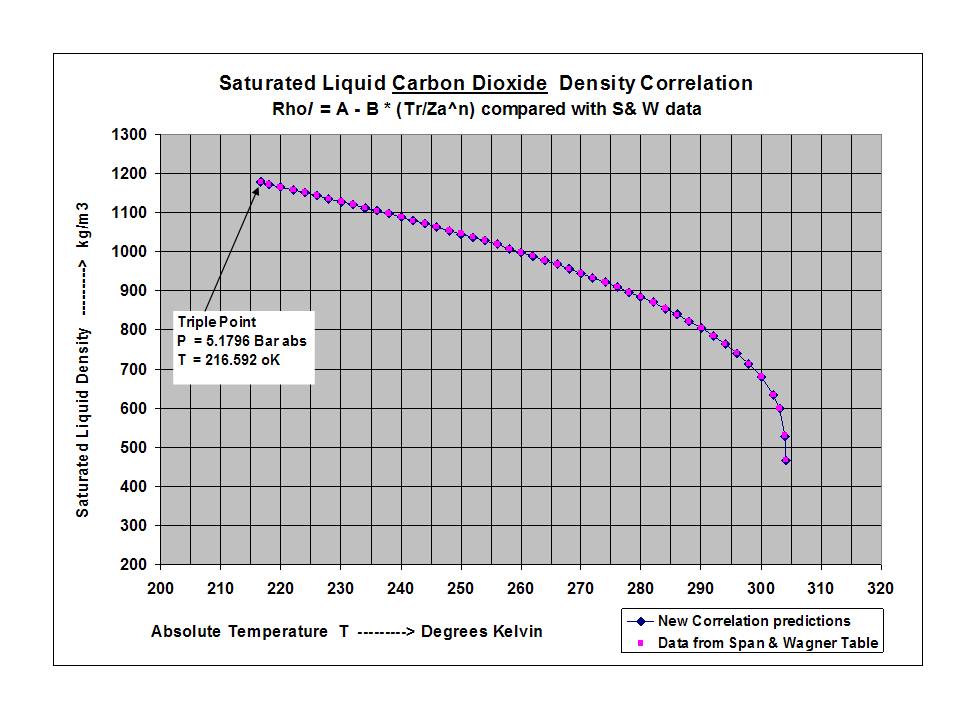
New compact Equations for the Compressibility Factor Z and Density of Liquid and Vapor Carbon Dioxide
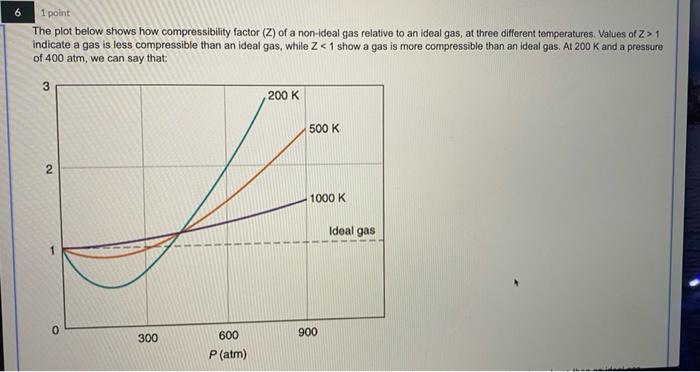
Solved 6 1 point The plot below shows how compressibility
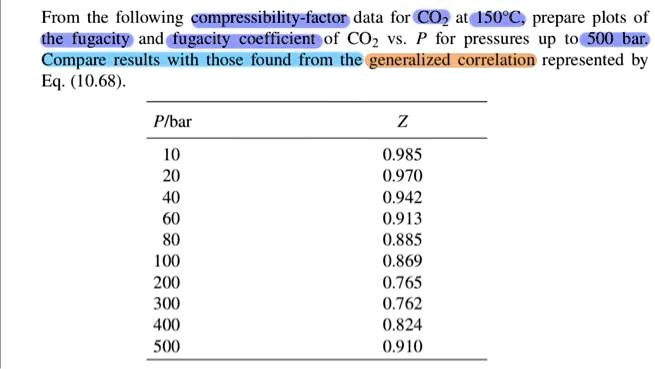
SOLVED: From the following compressibility-factor data for CO at 150°C, prepare plots of the fugacity and fugacity coefficient of CO vs. P for pressures up to 500 bar. Compare the results with

In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar

Energies, Free Full-Text







