An ideal gas is taken from (Pi, Vi) to (Pf, Vf) in three different ways. Identify the process in which the work done on the gas the most. - Physics

An ideal gas is taken from (Pi, Vi) to (Pf, Vf) in three different ways. Identify the process in which the work done on the gas the most.
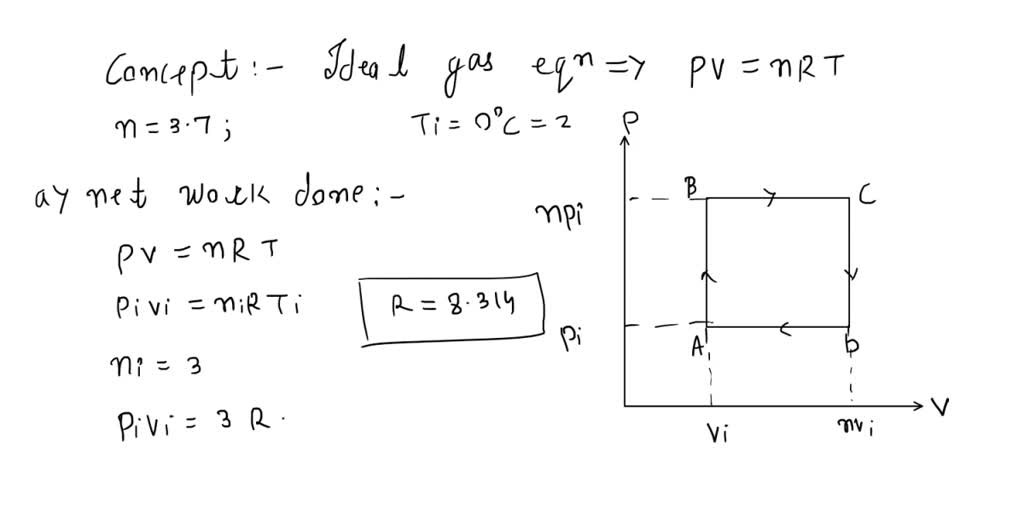
SOLVED: ideal gas initially at Pi, Vi, and Ti is taken through cycle as shown below: (Let the factor n 3.7.) nf Find the net work done on the gas per cycle

The origin of irreversibility and thermalization in thermodynamic processes - ScienceDirect

Can the Second law of thermodynamics be abandoned?

1st law

Untitled, PDF, Heat
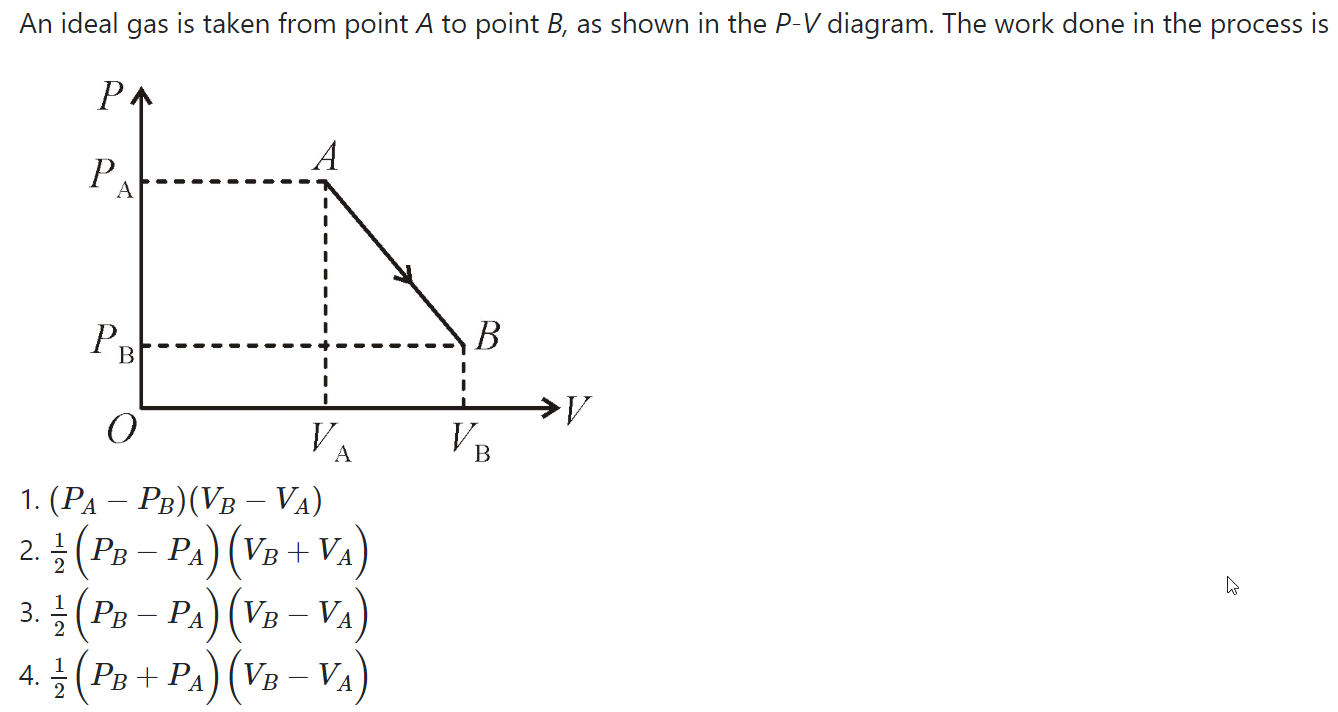
SOLVED: An ideal gas is taken from point A to point B, as shown in the P-V diagram. The work done in the process is 1. (PA-PB)(VB-VA) 2. (1)/(2)(PB-PA)(VB+VA) 3. (1)/(2)(PB-PA)(VB-VA) 4. (

i.e.16.10: A multi-step process - ppt download
Five moles of an ideal gas are compressed isothermally from A to B, as the graph illustrates. What is the work involved if the temperature of the gas is 307 K? Be

PPT - Gases, Heat, and Work PowerPoint Presentation, free download - ID:5076115

The Free High School Science Texts - James M. Hill Memorial High
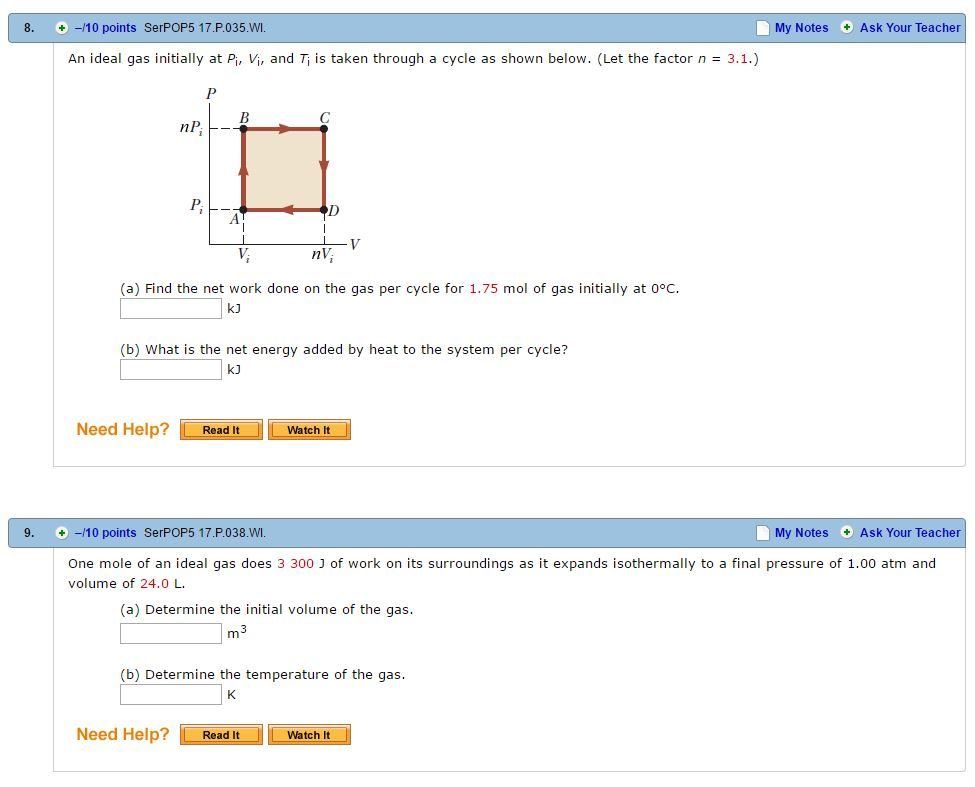
Solved An ideal gas initially at P_i, V_i, and T_i is taken
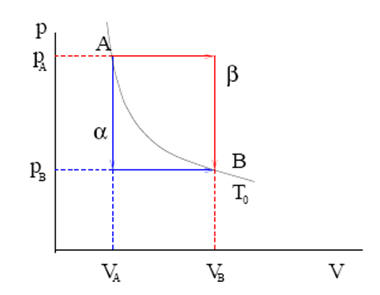
1st law