FDA says Medtronic MiniMed insulin pump recall is serious - MassDevice

The U.S. FDA has designated a recall of hundreds of thousands of Medtronic Minimed insulin pumps as Class I — the most serious type of recall. Medtronic (NYSE:MDT) first warned of safety problems with the pumps in November. The recall involves 322,005 pumps — MiniMed 630G (model MMT-1715) and MiniMed 670G (model MMT-1780) — in the […]

Drug Delivery Business News on LinkedIn: Baxter recalls some

Medtronic Diabetes Pump Lawsuit - MiniMed Insulin Pump
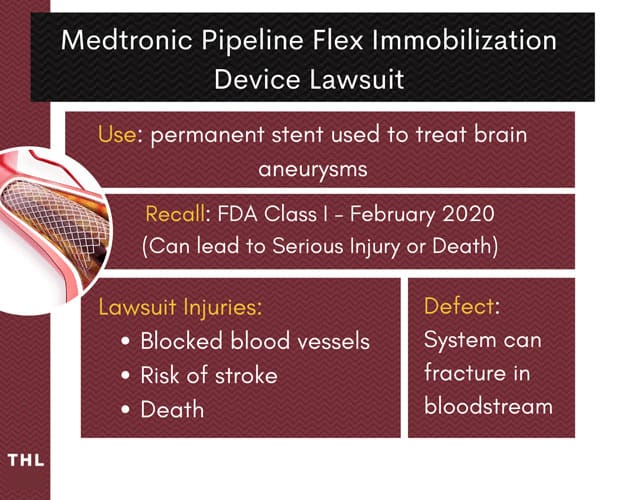
Medtronic Pipeline Flex Embolization Device Lawsuit

Medtronic recalls MiniMed insulin pumps after one death and many injuries - CBS News

Diabetes: Medtronic warns on insulin pump overdose
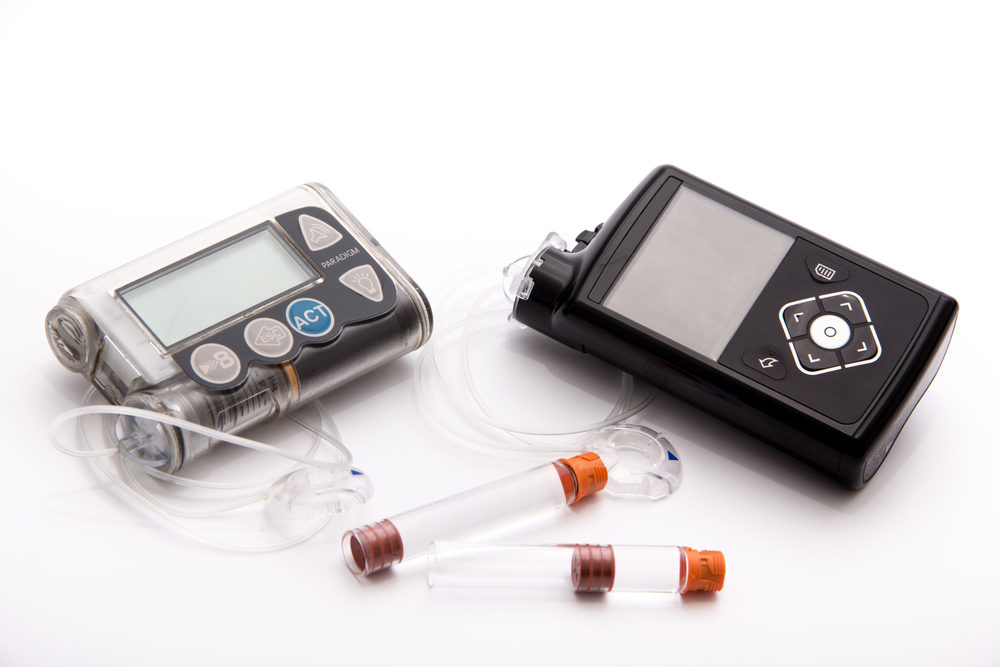
Medtronic Recalls More MiniMed Insulin Pumps for Dosage Issues

Medtronic Diabetes Pump Lawsuit - MiniMed Insulin Pump

Medtronic Recalls Some Insulin Pumps as FDA Warns They can be

Medtronic - Wikipedia

The worst catheter-based device recalls of 2020 - MassDevice

FDA recalls insulin pumps tied to over 2,000 injuries and 1 death

Medtronic CEO confirms FDA warning could affect approval timing

Medtronic MiniMed Insulin Pump Lawsuit

Medtronic expands 2 Class I recalls of MiniMed insulin pumps
Insulet has a Class I recall for the Omnipod 5 Android App