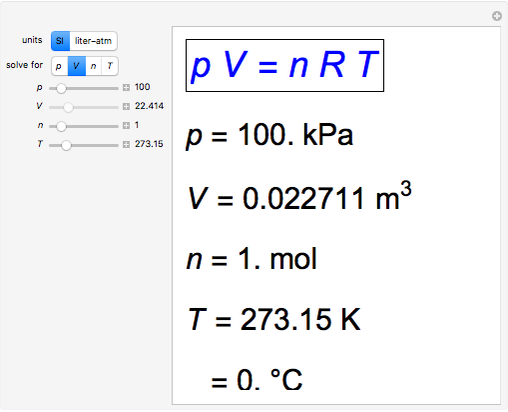
Ideal–Universal Gas Law
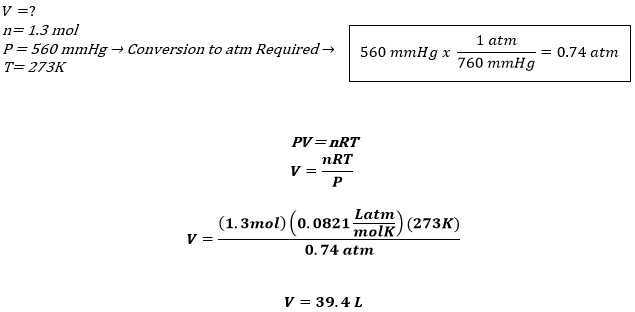
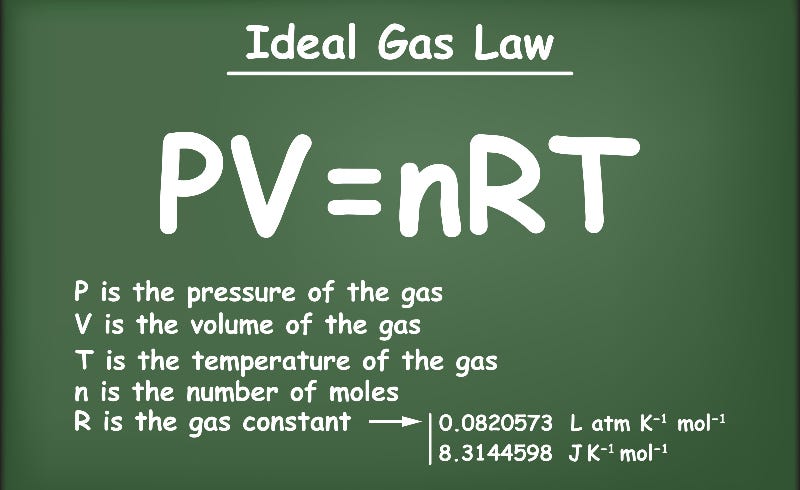
Definition: The Universal or Ideal Gas Law describes the relationship between all four properties (pressure, volume, number of moles, and temperature) as well as a gas constant called “R.” NOTE: The Ideal Gas Constant “R” has constant a value of 0.0821 L.atm/mol.K Relation: The relation between pressure (P) volume (V), number of moles (n) and…

SOLUTION: Ideal Gas law and Universal Gas Constant Questions and Answers - Studypool

83 Combined Gas Law Royalty-Free Photos and Stock Images
Ideal Gas Law - Wyzant Lessons

ANESTHESIA EQUIPMENT AND GAS LAW REVIEW - ppt download
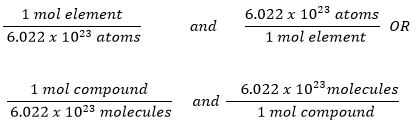
The Mole Concept: Molecules and Atoms

Forming Ions for Bonding

Forming Compounds

Combined Gas Law - Gas Laws explained
Units of Ideal Gas Constant R and Density of an Ideal Gas - video Dailymotion

Orbital Diagrams

Avogadro's Law

Calculating Standard Enthalpy of Reaction